About Oxford Immunotec
Oxford Immunotec is a global, commercial-stage diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. Our proprietary T-SPOT technology platform allows us to measure the responses of specific immune cells, known as T cells, to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases.
The initial product developed using T-SPOT technology platform is T-SPOT.TB test, which is used to test for latent Tuberculosis (TB) infection, or LTBI. T-SPOT.TB test has been approved for sale in over 50 countries, including the United States, where it has received pre-market approval (PMA) from the Food and Drug Administration (FDA), in Europe, where has obtained a CE mark, as well as Japan and China. T-SPOT.TB test has been included in clinical guidelines (that is, guidelines issued by governmental agencies and professional societies covering recommended or suggested uses of available diagnostics) for TB screening in 17 countries, including the United States, several European countries and Japan.
Product Overview
Oxford Immunotec’s first product based on the T-SPOT technology platform is the T-SPOT.TB test.
The T-SPOT.TB test is a revolutionary product that diagnoses both latent TB infection and active disease by measuring T cells that have been specifically activated by Mycobacterium tuberculosis (MTB) antigens.
The T-Cell Xtend reagent is an antibody complex that is added to blood samples in the laboratory immediately before running the T-SPOT.TB assay. It allows blood samples to be processed up to 32 hours after venepuncture without affecting the accuracy of the test.
T-SPOT.TB
The T-SPOT.TB test is a revolutionary product that diagnoses both latent TB infection and active disease by measuring T cells that have been specifically activated by Mycobacterium tuberculosis (MTB) antigens.
The T-SPOT.TB test sets new clinical standards of sensitivity and reliability. The product was licensed across Europe in July 2004, received FDA premarket approval in July 2008, was approved in China in 2010 and in Japan in 2012. It has been designed to replace the tuberculin skin test (Mantoux test), bringing effective TB testing to many new patient groups where the skin test gives poor or unreliable results.
This revolutionary technology has been tested in many thousands of patients in all parts of the world. Studies have shown the excellent sensitivity and specificity of the T-SPOT.TB test and demonstrate its utility for the diagnosis and control of TB (refer to clinical data).
The T-SPOT.TB kit is supplied with a plate containing 12 X 8 well strips. 4 wells are required for each patient sample.
How It Works
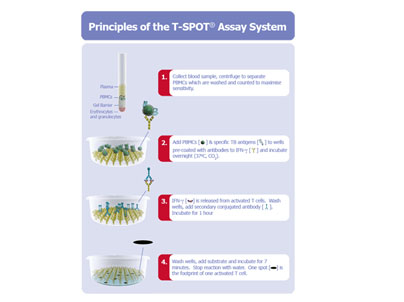
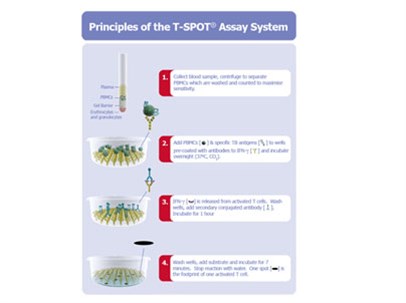
Principles of the T-SPOT Assay
The principles of our T-SPOT assay system, are shown below, using blood as the bodily fluid in the example. The process starts with a blood sample, from which PBMCs (specifically Peripheral Blood Mononuclear Cells) or WBC components containing T cells are separated, washed and counted. A pre-determined number of PBMCs and antigens specific to the disease or condition of interest are then added to the wells of a microtiter plate to which antibodies to interferon-gamma, or IFN-γ, are bound. The test is based on the principle that the T cells of an individual who carries an active infection will respond to the antigens and secrete interferon-gamma. The secretion of interferon-gamma by the T cells of the subject is captured by the anti-interferon-gamma antibodies coated to the floor of each well. The numbers of individual reacting T cells are enumerated through visualizing the footprint of each T cell by this secretion of interferon-gamma.
For more info, please visit: www.oxfordimmunotec.com